Suspension of use and sales
In the United States, there have been cases of people losing sight or dying after using artificial tears from a certain pharmaceutical company. Sales of these products are currently banned.
On the 21st (local time), the US Centers for Disease Control and Prevention (CDC) announced that users of the pharmaceutical company ‘Global Pharma HealthCare’ eye drops were infected with carbapenem-resistant Pseudomonas aeruginosa (VIM-GES-CRPA) one after another. The products in question are ‘EzriCare artificial tears’ and ‘Delsam Pharma ointment’. These products are eye drops that can be easily purchased at online shopping malls such as Amazon and eBay.
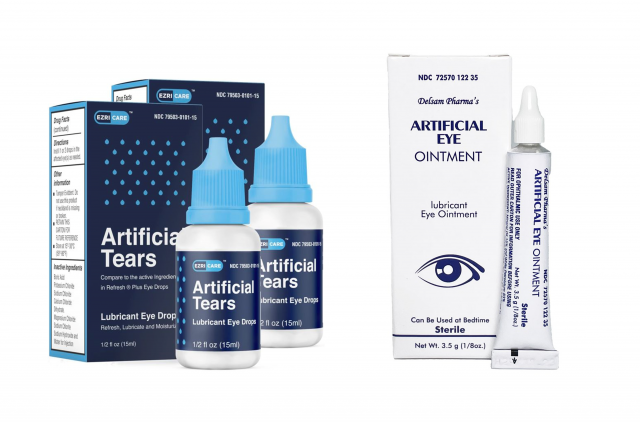 EzriCare artificial tears (left) and Delsam Pharma ointment from Global Pharma Healthcare, which the U.S. Food and Drug Administration (FDA) notified to stop using and selling. Amazon capture
EzriCare artificial tears (left) and Delsam Pharma ointment from Global Pharma Healthcare, which the U.S. Food and Drug Administration (FDA) notified to stop using and selling. Amazon captureSo far, 68 users in 16 states in the United States are known to have been infected. Of these, three died and eight lost sight. Four had their eyes removed, the CDC said.
The U.S. Food and Drug Administration (FDA) ordered the company to recall the product, saying that it may have been contaminated with antibiotic-resistant bacteria. In addition, the distributor notified the suspension of sales. CDC is investigating why the infection occurred by analyzing the products that were in production.
The CDC said, “You should stop using Global Pharma Healthcare’s eye drop products until further guidance is given.” do it,” he asked.
Pseudomonas aeruginosa is a pathogenic bacterium that is easily found in the natural environment of living spaces. The main symptom of an infection is yellow or green pus and a clear discharge from the eye. Eye pain, redness, and foreign body sensation are also symptoms. If your vision suddenly appears blurry after using eye drops or your sensitivity to light becomes sensitive, you may suspect an infection.
Lee Ye-ji,
Source: Donga
Mark Jones is a world traveler and journalist for News Rebeat. With a curious mind and a love of adventure, Mark brings a unique perspective to the latest global events and provides in-depth and thought-provoking coverage of the world at large.